The reaction of aniline forming diphenylamine and ammonia is a heterogen reaction where the reaction occurs in the gas phase with the help of a solid metal oxide catalyst is mainly alumina. Reaction mechanism is the reactants must be adsorbed on the catalyst surface, after the reaction occurs at the surface of the catalyst, the desorption substances from the surface of the catalyst and returned to the gas stream.
Reactions that occur following equation as follows:
Reaction rate:
rA= k.CA
rA = rate of reaction , kmole/kg cat.h
CA = concentration of aniline , kmole/m3
k = overall reaction rate constants , m3 /kg cat.h
We obtain the necessary data contained in U.S. Pat. 3118944, after being processed then the value will be the reaction rate constants as a function of temperature as follows::
T = temperature, Kelvin
The reactor used is adiabatic Fixed bed Reactor that do not require cooling or heating means that the reaction was allowed to happen as it is. Although the exothermic reaction (heat out), but the heat that arises is not large enough to increase the reaction temperature remained still in the allowed temperature range.
If required U.S. Pat. 3118944, can be downloaded by clicking here
The reactor used is adiabatic Fixed bed Reactor that do not require cooling or heating means that the reaction was allowed to happen as it is. Although the exothermic reaction (heat out), but the heat that arises is not large enough to increase the reaction temperature remained still in the allowed temperature range.
Data:
☺ the operating conditions
Temperature : 435-500°C
Pressure : ± 7.5 atm
Reactions heat : exothermic
Process conditions : adiabatic
Pressure : ± 7.5 atm
Reactions heat : exothermic
Process conditions : adiabatic
☺ Catalyst
Type : Alumina Al2O3
Shape : cylindrical
Size : 1/8 in x 1/8 in
Bulk density, rB : 650 lb/ft3
Particle density, rP : 1200 kg/m3
Arrangement of Differential Equations
If: x = C6H5NH2 conversion react
Arrangement of Differential Equations
If: x = C6H5NH2 conversion react
The composition of the mass in the reactor any time:
Component
|
initial
|
Current
|
C6H5NH2
|
Fao
|
Fa = Fao - Fao.x
|
(C6H5) 2NH
|
Fbo
|
Fb =Fbo+½.Fao.x
|
NH3
|
Fco
|
Fc = Fco+½.Fao.x
|
C6H5NO2
|
Fdo
|
Fd = Fdo
|
Total
|
Fto
|
Ft = Fto
|
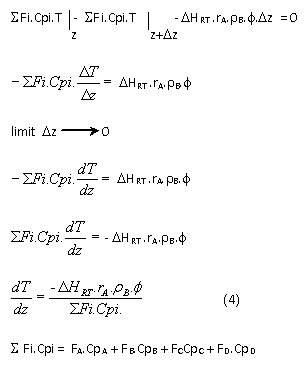
1. Mass balance of reactor
C6H5NH2 mass balance in the reactor in a volume elementRate of mass input – rate of mass output - rate of reaction = rate of accumulation
Mole C6H5NH2 any time :
Fa = Fao - Fao.x
2. Heat balance of reactor
In a volume element:
Rate of heat input – rate of heat output - rate of reaction heat = rate of accumulation
ΔHRT = heat of reaction as a function of temperature
Heat of reaction
Heat of formation of compounds at°C, ∆Hf 25 (Reid, R.C, 1977)
Component
|
ΔHf (kcal/mole)
|
C6H5NH2
(C6H5) 2NH
NH3
|
20.76
31.07
-10.92
|
Gas heat capacity, kcal/Kmole.K :
Data from (Reid, R.C., 1977) cp = a’ + b’.T + c’.T2 + d’.T3 ; is converted into
cp = a + b.T
component
|
a
|
B
|
C6H5NH2
|
7.2898
|
0.065673
|
(C6H5) 2NH
|
5.7820
|
0.122340
|
NH3
|
6.2399
|
0.007591
|
C6H5NO2
|
5.0223
|
0.069627
|
Heat of reaction is calculated based on heat of formation of products and reactants
Reaction
2 C6H5NH2 --------> (C6H5)2NH + NH3
∆Hr25 = ∑∆Hf products - ∑∆Hf reactants
∆Hr25 = ∆Hf (C6H5)2NH + ∆Hf NH3 – ∆Hf C6H5NH2
= 31.07 + (–10.92) - 2. (20.76)
= -21.37 kcal/2 mole
= -21370 kcal/2 kmole C6H5NH2
= -10685 kcal/ kmole C6H5NH2
Heat of reaction at temperature T:
(C6H5)2NH NH3 2 C6H5NH2 Δcp
Δa: 5.782 + 6.2399 - 2 (7.2898) = -2.5577
Δb: 0.12234 + 0.007591 - 2 (0.065673) = -1.4153.10-3
|
Δcp = (-2.5577 - 1.4153.10-3 T) kcal/ kmole.K
= - 2.5577 (T-298) - 7.0765.10-4 (T2 -2982) kcal/2 kmole C6H5NH2
= - 2.5577 (T-298) - 7.0765.10-4 (T2 -2982) kcal/2 kmole C6H5NH2
= - 1.27885 (T-298) - 3.538.10-4 (T2 -2982) kcal/kmole C6H5NH2
Then:
ΔHrT = -10685 - 1.27885 (T-298) - 3.538.10-4 (T2 -2982) kcal/kmole
3. Pressure drop
Pressure drop is calculated using equation (5-196) Perry 1984:
With:
fm = friction factor, function of Reynolds number
n = exponential, function of Reynolds number
Dp = equivalent diameter of catalyst, m
ε = voidage, empty volume fraction
φ = particle shape factor
G = mass velocity of fluid is based on an empty tube (kg/h.m2)
ρ = fluid density, kg/m3
gc = 9.8 m/s2
Looking for a unit dP/dz be obtained:
The catalyst bed thickness calculation
to calculate the catalyst bed thickness in the reactor in order to obtain the desired conversion then use the following equations:
To obtain the desired conversion then 3 of the above differential equations solved simultaneously by using existing mathematical methods and computer programs are made.
Completion Ordinary Differential Equations (ODE) can be solved using the method:
1.Metode Euler (explicit), also called the method of integration of initial value
2.Metode Modified Euler (implicit) called the Predictor Corrector Method or Heun method
3.Metode Runge-Kutta of order of four
Step by step of calculation:
1. Determine the initial condition into the reactor at z = 0 m: x, T, P, Δz
2. Calculated heat of reaction and reaction rate.
3. Calculated the value of :
4. Calculation is continued until the desired conversion.
The calculations above are done by trying variables that can be varied as follow: Di, To and P
Another equation is needed physical properties as function of temperature:
(Carl L. Yaws, 1999)
Component
|
BM
|
C6H5NH2
|
93.129
|
(C6H5) 2NH
|
169.227
|
NH3
|
17.031
|
C6H5NO2
|
123.113
|
Gas viscosity
Gas viscosity as function of temperature in the table 21-1 and 21-2 Carl L. Yaws, 1999:
µ = A + B.T + C.T²
µ = viscosity, µP (micro poise)
T = Kelvin
1 µP = 0.00036 kg/h.m
A, B and C constanta as follows :
component
|
A
|
B
|
C
|
C6H5NH2
|
-19.148
|
0.3067
|
-5.3256E05
|
(C6H5) 2NH
|
-21.162
|
0.2544
|
-4.5847E-05
|
NH3
|
-7.874
|
0.3670
|
-4.470E-06
|
C6H5NO2
|
-19.512
|
0.2958
|
-5.1687E-05
|
Viscosities of gas mixture
µm = ∑ µi,yi Feed composition into the reactor by mass balance calculation is as follows:
µm = ∑ µi,yi Feed composition into the reactor by mass balance calculation is as follows:
Component
|
Kg/h
|
Kmole/h
|
C6H5NH2
|
19654.32
|
211.0441
|
(C6H5) 2NH
|
63.13
|
0.4727
|
C6H5NO2
|
58.19
|
0.3731
|
Total
|
19775.65
|
211.8898
|
Quantity that are calculated
Quantity that counted in the calculation of the reactor is:
1). Changed in conversion
2). Changes in reaction temperature
3). Changes in pressure drop in the reactor
The program is run using QuickBasic (QB) or Scilab.
The results run the program will be obtained
relationship between the conversion to catalyst bed thickness, reaction temperature to catalyst bed thickness and mass composition as follows:
------------------------------------------------------------------------------------
z(m) X FA FB FC T(°C) P(atm)
------------------------------------------------------------------------------------
0.00 0.0000 211.044 0.373 0.000 435.0 7.500
0.04 0.0020 210.612 0.589 0.216 435.4 7.498
0.08 0.0041 210.177 0.807 0.434 435.9 7.496
0.12 0.0062 209.738 1.026 0.653 436.3 7.494
0.16 0.0083 209.295 1.247 0.874 436.7 7.491
0.20 0.0104 208.849 1.471 1.098 437.2 7.489
0.24 0.0125 208.399 1.695 1.322 437.6 7.487
0.28 0.0147 207.946 1.922 1.549 438.1 7.485
0.32 0.0168 207.488 2.151 1.778 438.5 7.483
0.36 0.0190 207.027 2.382 2.009 439.0 7.481
0.40 0.0212 206.562 2.614 2.241 439.5 7.479
0.43 0.0235 206.093 2.849 2.476 439.9 7.476
0.47 0.0257 205.619 3.085 2.712 440.4 7.474
0.51 0.0280 205.142 3.324 2.951 440.9 7.472
0.55 0.0302 204.661 3.565 3.192 441.3 7.470
0.59 0.0325 204.175 3.807 3.434 441.8 7.468
0.63 0.0349 203.686 4.052 3.679 442.3 7.466
0.67 0.0372 203.192 4.299 3.926 442.8 7.463
0.71 0.0396 202.693 4.549 4.175 443.3 7.461
0.75 0.0420 202.190 4.800 4.427 443.8 7.459
0.79 0.0444 201.683 5.054 4.681 444.3 7.457
0.83 0.0468 201.171 5.310 4.937 444.8 7.455
0.87 0.0492 200.654 5.568 5.195 445.3 7.452
0.91 0.0517 200.133 5.829 5.456 445.8 7.450
0.95 0.0542 199.606 6.092 5.719 446.3 7.448
0.99 0.0567 199.075 6.357 5.984 446.9 7.446
1.03 0.0593 198.539 6.625 6.252 447.4 7.444
1.07 0.0618 197.998 6.896 6.523 447.9 7.441
1.11 0.0644 197.452 7.169 6.796 448.5 7.439
1.15 0.0670 196.901 7.445 7.072 449.0 7.437
1.19 0.0697 196.344 7.723 7.350 449.6 7.435
1.22 0.0723 195.783 8.004 7.631 450.1 7.432
1.26 0.0750 195.215 8.287 7.914 450.7 7.430
1.30 0.0777 194.642 8.574 8.201 451.2 7.428
1.34 0.0805 194.064 8.863 8.490 451.8 7.426
1.38 0.0832 193.480 9.155 8.782 452.4 7.424
1.42 0.0860 192.890 9.450 9.077 453.0 7.421
1.46 0.0888 192.294 9.748 9.375 453.5 7.419
1.50 0.0917 191.692 10.049 9.676 454.1 7.417
1.54 0.0946 191.084 10.353 9.980 454.7 7.415
1.58 0.0975 190.470 10.660 10.287 455.3 7.412
1.62 0.1004 189.850 10.970 10.597 455.9 7.410
1.66 0.1034 189.223 11.283 10.910 456.5 7.408
1.70 0.1064 188.590 11.600 11.227 457.2 7.406
1.74 0.1094 187.950 11.920 11.547 457.8 7.403
1.78 0.1125 187.304 12.243 11.870 458.4 7.401
1.82 0.1156 186.651 12.570 12.197 459.1 7.399
1.86 0.1187 185.990 12.900 12.527 459.7 7.396
1.90 0.1219 185.323 13.234 12.861 460.3 7.394
1.94 0.1251 184.648 13.571 13.198 461.0 7.392
1.98 0.1283 183.966 13.912 13.539 461.7 7.390
2.01 0.1316 183.277 14.256 13.883 462.3 7.387
2.05 0.1349 182.580 14.605 14.232 463.0 7.385
2.09 0.1382 181.876 14.957 14.584 463.7 7.383
2.13 0.1416 181.163 15.313 14.940 464.4 7.380
2.17 0.1450 180.443 15.674 15.301 465.1 7.378
2.21 0.1485 179.714 16.038 15.665 465.8 7.376
2.25 0.1519 178.978 16.406 16.033 466.5 7.373
2.29 0.1555 178.232 16.779 16.406 467.2 7.371
2.33 0.1590 177.479 17.156 16.783 468.0 7.369
2.37 0.1627 176.716 17.537 17.164 468.7 7.366
2.41 0.1663 175.945 17.923 17.550 469.5 7.364
2.45 0.1700 175.164 18.313 17.940 470.2 7.362
2.49 0.1738 174.375 18.708 18.335 471.0 7.359
2.53 0.1775 173.576 19.107 18.734 471.7 7.357
2.57 0.1814 172.767 19.511 19.138 472.5 7.355
2.61 0.1852 171.949 19.920 19.547 473.3 7.352
2.65 0.1892 171.121 20.335 19.962 474.1 7.350
2.69 0.1931 170.283 20.754 20.381 474.9 7.347
2.73 0.1972 169.434 21.178 20.805 475.7 7.345
2.77 0.2012 168.575 21.607 21.234 476.6 7.343
2.80 0.2054 167.706 22.042 21.669 477.4 7.340
2.84 0.2095 166.825 22.482 22.109 478.2 7.338
2.88 0.2137 165.934 22.928 22.555 479.1 7.335
2.92 0.2180 165.031 23.380 23.007 480.0 7.333
2.96 0.2224 164.117 23.837 23.464 480.8 7.331
3.00 0.2267 163.191 24.300 23.927 481.7 7.328
3.04 0.2312 162.253 24.769 24.396 482.6 7.326
3.08 0.2357 161.303 25.244 24.871 483.5 7.323
3.12 0.2403 160.340 25.725 25.352 484.4 7.321
3.16 0.2449 159.365 26.212 25.839 485.4 7.319
3.20 0.2496 158.377 26.706 26.333 486.3 7.316
3.24 0.2543 157.376 27.207 26.834 487.3 7.314
3.28 0.2591 156.362 27.714 27.341 488.2 7.311
3.32 0.2640 155.334 28.228 27.855 489.2 7.309
3.36 0.2689 154.293 28.749 28.376 490.2 7.306
3.40 0.2739 153.237 29.277 28.904 491.2 7.304
3.44 0.2790 152.167 29.812 29.439 492.2 7.301
3.48 0.2841 151.082 30.354 29.981 493.3 7.299
3.52 0.2893 149.983 30.904 30.531 494.3 7.296
3.56 0.2946 148.868 31.461 31.088 495.4 7.294
3.59 0.3000 147.738 32.026 31.653 496.4 7.291
3.63 0.3054 146.592 32.599 32.226 497.5 7.289
3.67 0.3109 145.430 33.180 32.807 498.6 7.286
3.71 0.3165 144.252 33.769 33.396 499.7 7.284
3.75 0.3221 143.057 34.367 33.993 500.8 7.281
3.79 0.3279 141.846 34.972 34.599 502.0 7.279
3.83 0.3337 140.617 35.587 35.214 503.1 7.276
3.87 0.3396 139.371 36.210 35.837 504.3 7.274
3.91 0.3456 138.107 36.841 36.468 505.5 7.271
3.95 0.3517 138.101 36.844 36.471 506.7 7.268
--------------------------------------------------------------------------------------
Graphic relationship shown by the figure below:






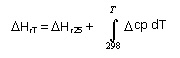








Tidak ada komentar:
Posting Komentar